 Dr. Carl M. Boyd
Dr. Carl M. Boyd Dr. Carl M. Boyd
Dr. Carl M. Boyd
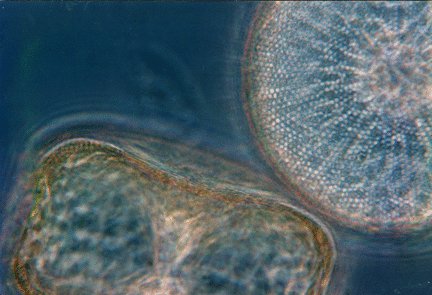

On the way to exploring this problem we first explored the basic electrophysiology of the marine diatom Coscinodiscus by using conventional two electrode voltage clamp techniques that allowed us to make several observations concerning flux and internal concentrations of various ions (see references below). Our experiments showed us that the gradient of potassium ions establishes the internal melieu of the cell, and that nitrate ions are moved into a diatom as co-transported ions, i.e, one nitrate ion is carried into the cell by the downhill movement of a species of cation. The name of thise cation is still unknown: in higher plants and many freshwater algae these nutrient ions are transported by the influx of protons, but the gradient of protons between the seawater environment and cytoplasm is almost nil and does not have sufficient energy to transport nitrate ions. We postulate that sodium ions provide the required energy gradient in the marine environment, and the proof of that proposition continues to motivate us.
One tool that we have developed in our electrophysiological research is the use of a saw-tooth voltage function that is imposed across the cell membrane of the cell being studied to generate voltage / current relationships. This function is an improvement over the use of conventional stepped pulses in that it quickly gives us information on several variables not otherwise obtained. Several publications have resulted from the development and use of this technique.
While we were studying ion flux in diatoms by electrophysiological means, other workers were isolating the genes responsible for the transport of nitrate . and the proteins associated with thegenes. from a variety of plant cells, including marine diatoms. We now know that the nitrate-transporting proteins are composed of chains of about 500 amino acids, and that individual protein molecules are inbedded in the cell membrane as 12 connected helices that are probably arranged to form a pore that functions to move nitrate into the cell along with its driving ions.
In order to have a cell-system that is less complex than a plant cell, we have pursued techniques that allow us to express these nitrate transporters in the relative simplicity of a frog.s egg. This is done by transcribing in vitro the DNA of the gene in question into RNA that is then injected into the unfertilized oocyte of the frog Xenopus laevis. Latent mechanisms of the oocyte translate the RNA into the nitrate-transporting protein that migrates to the cell wall of the oocyte, where it functions in a manner similar to its counterpart in a plant. We can study the kinetics of nitrate uptake in the oocyte by using our electrophysiological techniques, and we can attempt to understand how the protein actually works by altering the sequence and type of amino acids in the protein through genetic manipulations. To date we have not been able to express the nitrate-transporting genes from our diatoms (we are continuing our efforts), but we have had considerable success with a similar gene from the fungus Emericella (Aspergillus), and have prepared a manuscript now submitted.
It is possible that marine diatoms use two different proteins that, in conjunction, are able to move nitrate into the cell. This pattern is established in other plant systems and may exist in marine diatoms. We hope to detect this protein when the complete genomic sequence of the marine diatom Thalassiosira pseudonana is released this winter. The publication of this sequence will have a profound effect on our knowledge of marine phytoplankton and will be the basis of extensive, worldwide, research for several years to come.
These studies are beyond the scope of competence of a single individual and are the results of a team composed of myself, of Prof. Dietrich Gradmann who is a plant physiologist from the University of Gottingen, and of Dr. Jessica Boyd, a molecular microbiologist at the National Research Council laboratory in Halifax.
A study that derived from our initial interest in the ionic composition of marine algal cells explores how phytoplankton cells may achieve neutral buoyancy. Marine phytoplanktonic cells can achieve neutral buoyancy only if the excess density of their relatively heavy structural materials (proteins, carbohydrates, silicate) is compensated for by the incorporation of materials that have densities less than seawater. We calculated densities and osmotic concentrations for several marine algae, based on published values of structural materials and concentrations of inorganic ions and other osmolytes. The calculations, incorporating the partial molal volume, molecular mass, concentrations, and osmotic coefficients, indicate that most published listings of intracellular osmolytes in marine algae are insufficient to provide the turgor known to exist. Similarly, the density of phytoplanktonic cells, calculated on the basis of known or estimated concentrations of cellular components, generally exceeds the density of seawater which would cause negative buoyancy (sinking) throughout. We show that neutral buoyancy cannot be achieved solely by regulating concentrations of common inorganic ions as proposed by Gross and Zeuthen. We use models of osmotic concentration and cellular density in which we supplement known concentrations of osmolytes with proxy osmolytes. In particular, concentrations of some 100 mol.m-3 of quaternary ammonium derivatives can explain the deficits of both osmotic concentration and buoyancy.
Gradmann, D., and C. M. Boyd, 2004. Current-voltage-time records of ion translocating enzymes. Eur. Biophys. J. 33:396-411.
Boyd, J, D. Gradmann, and C. M. Boyd, 2003. Transinhibition and Voltage-gating in a Fungal Nitrate Transporter. J. Membrane Biol. 195:109-120.
Boyd, C.M. and D. Gradmann, 2002. Impact of osmolytes on the buoyancy of marine phytoplankton. Marine Biology 141:605-618.
Gradmann, D. and C.M. Boyd. 2000 Three types of membrane excitations in the marine diatom Coscinodiscus wailesii. J. Membrane Biol. 175:149-160.
Gradmann, D. and C.M. Boyd, 1999. Electrophysiology of the marine diatom Coscinodiscus wailesii. IV: Types of non-linear current-voltage-time relationships recorded with a single saw-tooth voltgage clamp. European Biophysics J. 28: 591-599.
Boyd, C.M. and D. Gradmann, 1999. Electrophysiology of the marine diatom Coscinodiscus wailesii. I: Endogenous changes of membrane voltage and resistance. Journal of Experimental Botany. 50:445-451.
Gradmann, D. and C.M. Boyd 1999. Electrophysiology of the marine diatom Coscinodiscus wailesii. II: Potassium currents. Journal of Experimental Botany. 50:452-459.
Boyd, C.M. and D. Gradmann, 1998. Electrophysiology of the marine diatom Coscinodiscus wailesii. III: Uptake of nitrate and ammonium. Journal of Experimental Botany. 50:461-467.
Gradmann, D. and C.M. Boyd, 1995. Membrane voltage of marine phytoplankton, measured in the diatom Coscinodiscus. Marine Biology 123:645-650.
Other studies dealing with the feeding of Antarctic krill and the general biology of other zooplankton were published prior to 1995.